Arpita Vyas’s current research elucidates the mechanisms of sex-specific cardiovascular dysfunction due to the prenatal exposure of excess androgens. In 2020, she secured a multimillion-dollar R01 grant from the National Institutes of Health (NIH) and National Heart Lung and Blood Institute titled “Gestational Hyperandrogenism in Cardiovascular Programming.” The research endeavor has expertise from the University of Michigan and Michigan State University.
Overall, her research endeavors encompassing bench-to-bedside and back-to-bench approach addressing both maternal-fetal medicine, cardiology, and endocrinology provides valuable research opportunities for medical students, undergraduates and graduate students interested in the field of maternal-fetal medicine, cardiology and pediatric medicine.
- Sex-specific impact of early programming of cardiac morphology by prenatal Testosterone (T) excess and relate it to temporal changes in cardiac function across the life span.
- The epigenetic and transcriptional mechanisms underlying the sex-specific impact of prenatal T excess on cardiac remodeling
- Determine if early sex-specific perturbations induced by fetal exposure to excess T that lead to cardiac remodeling are mediated via androgenic or insulin signaling pathways
Sex differences in cardiovascular programming with gestational hyperandrogenism
Cardiovascular disease (CVD) remains the leading cause of death worldwide. Sex differences exist in both prevalence and burden of CVD. Despite it being the leading cause of death in both men and women, men have a higher age-adjusted rate of CVD mortality. Despite significant advances in identifying CVD risk factors and their therapeutics, the morbidity and mortality from CVD remain high. Integration of sex/gender as a biological variable has become an essential requirement in clinical and animal studies including those in the field of cardiovascular research. In this regard, determining the early histological and molecular alteration in the LV of both males and females from prenatal T excess allows designing sex-specific early interventions to prevent CVD onset.
Utilizing a novel sheep model of gestational hyperandrogenism, our laboratory is investigating how perturbed in-utero environment with testosterone excess leads to sex differences in cardiovascular health in the offspring.
Our recent work showed sex differences in cardiac morphology and molecular pathways during mid gestation in offspring.
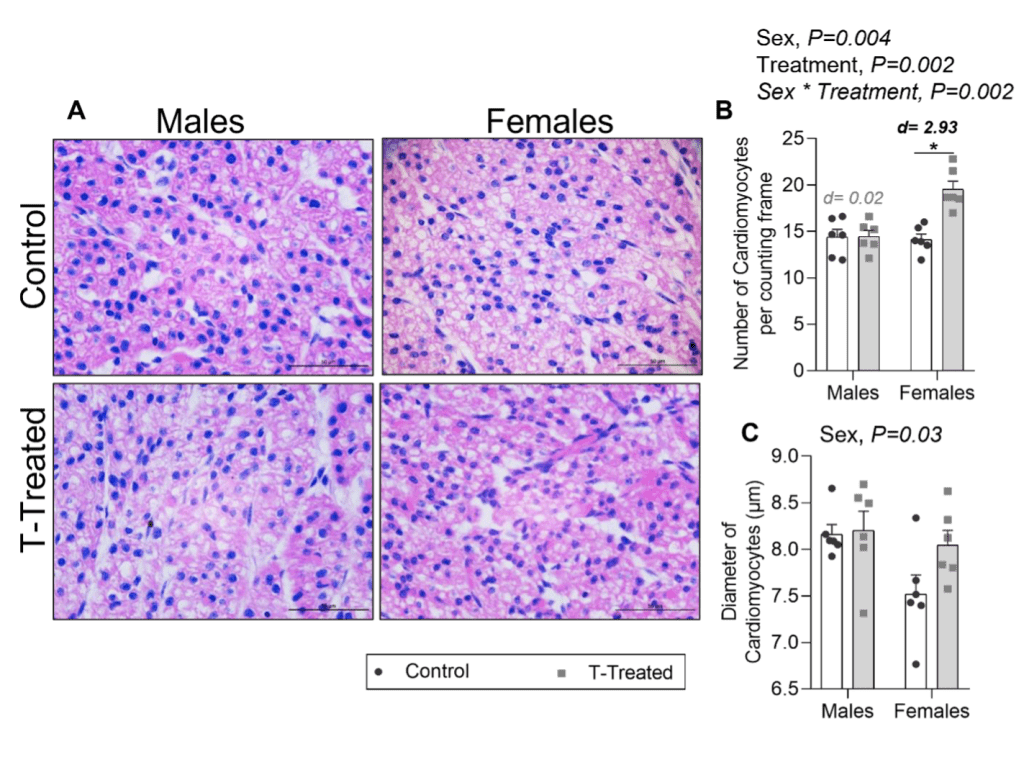
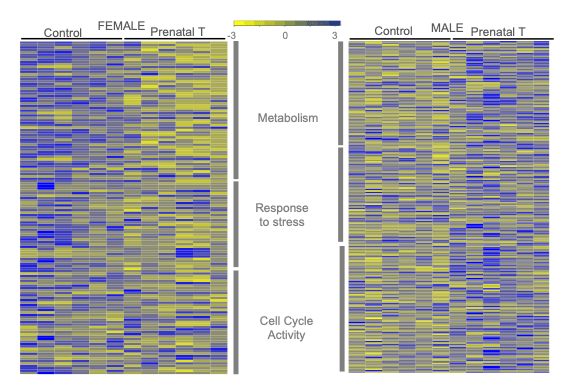
Maternal cardiovascular programming with gestational hyperandrogenism
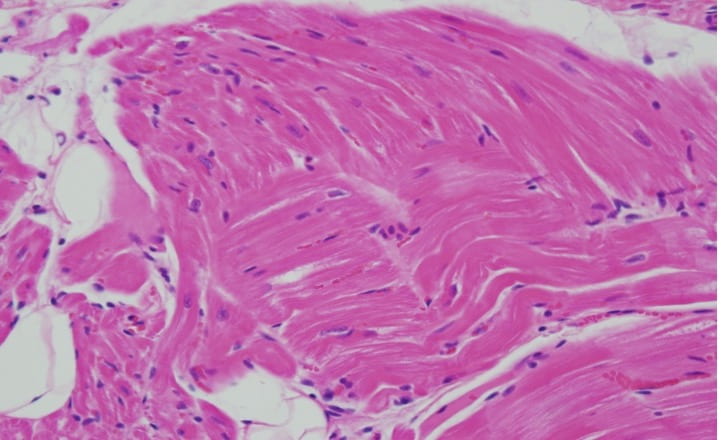
Greater than 19 million deaths in 2020 were attributed to CVD globally with approximately 1 million occurring in the US. Roughly half of these deaths (441,532) were in women, making it the leading cause of death for women in the U.S. in 2020. Interestingly, over a third of all maternal deaths are due to cardiovascular disease (CVD). Moreover, over half of all maternal deaths occur in postpartum period. Our lab is studying the impact of gestational hyperandrogenism on maternal cardiovascular health utilizing the sheep model of pregnancy. We note increase in cardiac collagen deposition in maternal left ventricle tissue at three months postpartum.
We are currently exploring the molecular pathways underlying such changes.
Cardiovascular programming with endocrine disrupting chemicals
Since the 1950’s greater than 140,000 new chemicals are being produced globally. This has inevitably led to increased numbers and amount of chemicals released into the air, sea and soil which contribute to environment pollution. Environmental endocrine disrupting chemicals (EDCs), that are non-biodegradable and exhibit both bioaccumulation and biomagnification and it is widely accepted that such anthropogenic EDCs are a significant global public health concern. EDCs can disrupt cardiometabolic health through modification of endocrine regulation via their estrogenic or androgenic properties. The exact underlying mechanisms by which EDCs affect metabolic and cardiovascular health have not been elucidated fully, but accumulating evidence implicates sexually-dimorphic molecular and epigenetic programming of genes that regulate these physiological processes.
Our lab is exploring the impact of EDC mixtures on cardiovascular health of the offspring.